SIGN UP FOR MORE INFORMATION
Get the latest communications and product updates from Organogenesis.
When you’re looking for a complete dehydrated placental allograft to support wound management, give your patients NuShield®. Convenience without compromise, NuShield provides a protective barrier and extracellular matrix (ECM) scaffold to protect wounds, providing an optimal environment for healing.
Note: NuShield retains all layers of the amnion/chorion membrane. Why NuShield?Preservation methods vary among placental allografts and affect the final product characteristics.
Learn about preservation methodsSee a retrospective case study showing how NuShield helped in managing a variety of wound types.
NuShield is a shelf-stable, dehydrated placental allograft that undergoes a unique preservation method.
See how NuShield helps to protect wounds, providing an optimal environment for healingNuShield uses the novel preservation method LayerLoc™ to retain all native layers. As a protective barrier and ECM scaffold, NuShield helps protect wounds, providing an optimal environment for healing.
See the tissue composition of NuShieldTalk to an Organogenesis Tissue Regeneration Specialist about the more complete dehydrated placental allograft tissue product today.
Contact usNuShield can be used as a protective barrier in a variety of clinical applications with flexibility for various wound types from head to toe.
View product informationNuShield was used to manage real-world patients in a retrospective case series.
See the results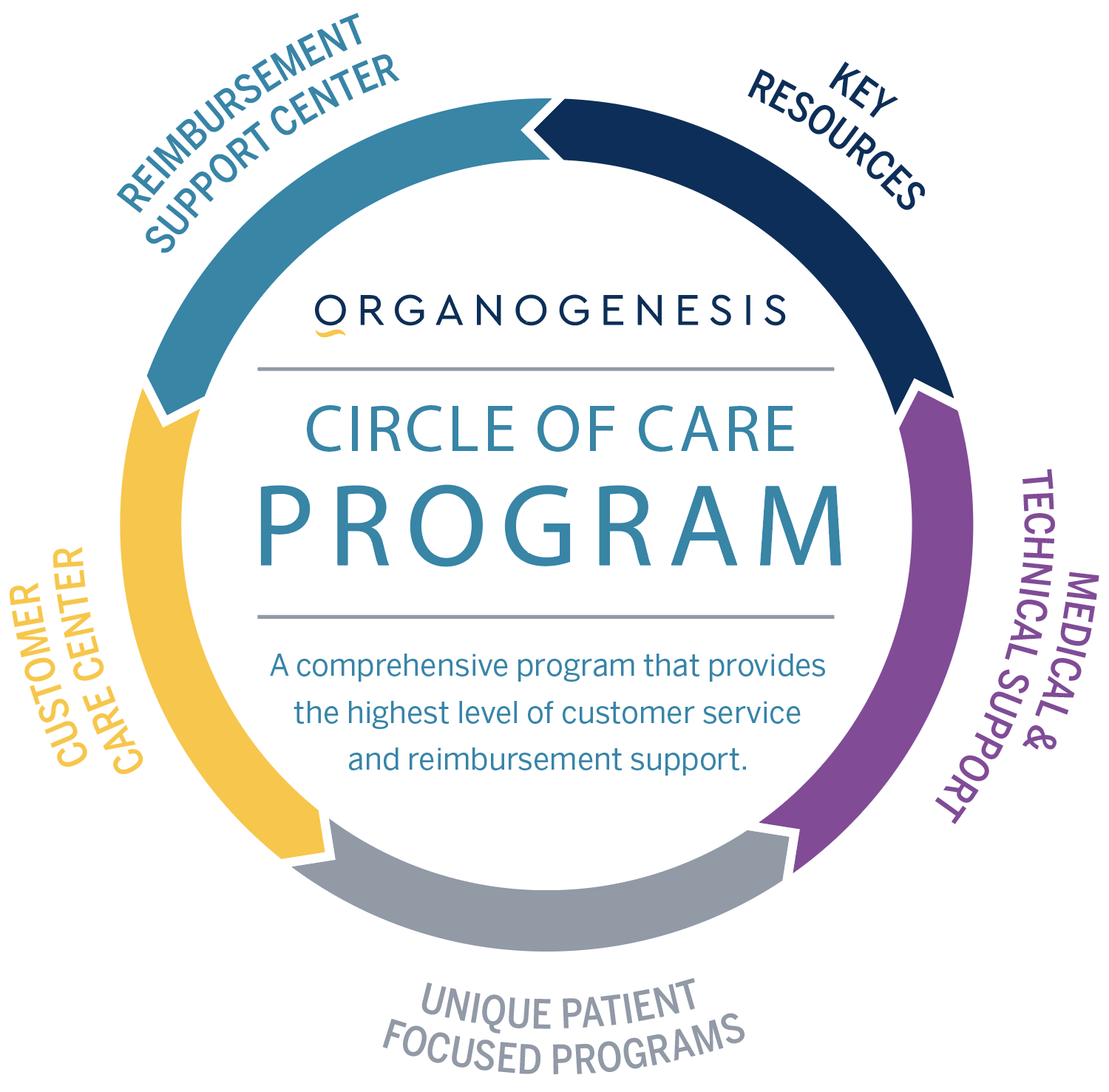
Organogenesis' Circle of Care is a comprehensive program that provides the highest-quality customer service and reimbursement support. The program’s wide range of expertise includes:
Please refer to the NuShield instructions for use for usage and safety information.
References: Data on File, Organogenesis Inc. Supporting information available upon request.